Scientific Posters
Download scientific posters from past events and conferences
CASSS 2025

POSTERImpact of internal fragment ion identification on oligonucleotides scoring metric
Enhancing Discrimination Power in Oligonucleotide Mass Spectrometry Through Internal Fragment Analysis
POSTERComprehensive isoform annotation and quantitation in DIA data
Achieving Superior Sensitivity and Precision in DIA Protein Quantification
POSTERProgressive Deconvolution for Robust Intact MS Analysis
Beyond Conventional Deconvolution: Tackling Complex Biologics and ADCs
POSTERAdvancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Streamline host cell protein discovery with automated quantitation, protein-centric analysis, and interactive dashboards for faster decision-making
POSTERAdvancements in Data Review for Therapeutic Protein Glycosylation Monitoring with Deep Query Powered Dashboards
Byosphere's deep query dashboards unify glycan data, enabling real-time insights, trend analysis, and streamlined therapeutic protein monitoring.

POSTERComprehensive Characterization of Disulfide Linkages in a Cysteine-Engineered Bispecific Antibody Using EThcD-on a modified Orbitrap Hybrid Mass Spectrometry
Advanced ETThcD-MS Workflow for Comprehensive Disulfide Bond Mapping
ASMS 2025
POSTERASMS 2025 - Comprehensive isoform annotation and quantitation in DIA data
New Byos search engine improves isoform detection, quantitation precision, and match confidence in complex DIA proteomics workflows.
POSTERASMS 2025 Digested Oligonucleotide IP-RPLC-MS/MS Characterization of mRNA Sequence, 5’ Cap, and 3’ PolyA Tail
Map mRNA sequence, cap, and tail in a single LC-MS/MS assay
POSTERASMS 2025 Protein-Centric Quantitation with Byos and GraphPad Prism
This poster highlights a protein-centric workflow combining Byos® MPQ with GraphPad Prism to reveal significant trends in complex proteomic datasets.
POSTERASMS 2025 Inter-Lab Comparison of MAM Data Using Byosphere
See how labs across Japan used Byosphere® to analyze MAM data on NISTmAb and uncover differences in sample prep, MS parameters, and CQA quantitation.

POSTERASMS 2025 Advancing CHO Proteomics with Multi-Protein Quantitation Workflows
This poster presents an MPQ-based workflow for high-throughput CHO proteome analysis, enabling better sensitivity, quantitation, and data inspection.
POSTERAdvancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Streamline host cell protein discovery with automated quantitation, protein-centric analysis, and interactive dashboards for faster decision-making
TIDES 2025

POSTERTides 2025 Digested Oligonucleotide IP-RPLC-MS/MS Characterization of mRNA Sequence, 5’ Cap, and 3’ PolyA Tail
Map mRNA sequence, cap, and tail in a single LC-MS/MS assay
PEGS 2025
POSTERPEGS 2025 - Orthogonal techniques for LCMS characterization of a lysine-conjugated ADC
Orthogonal LC-MS methods simplify lysine-conjugated ADC analysis for precise DAR calculation and site occupancy insights
POSTERPEGS 2025 - Advancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Streamline host cell protein discovery with automated quantitation, protein-centric analysis, and interactive dashboards for faster decision-making

POSTERPEGS 2025 - Dedicated computational methods for feature detection and quantification in large molecule mass spectrometry analyses
Progressive deconvolution enables precise detection and quantification of complex biologics across highly heterogeneous samples
HUPO 2025
POSTERAdvancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Introducing the new multi-protein quantitation mass spectrometric analysis workflow tools for discovery-phase protein scientists.
WCBP 2025

POSTERAdvancements in Data Review for Therapeutic Protein Glycosylation Monitoring with Deep Query Powered Dashboards
Byosphere's deep query dashboards unify glycan data, enabling real-time insights, trend analysis, and streamlined therapeutic protein monitoring.

POSTERAdvancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Introducing the new multi-protein quantitation mass spectrometric analysis workflow tools for discovery-phase protein scientists.
SLAS 2025

POSTERA Fully Integrated and Automated Platform for Screening Biologics
An end-to-end automated workflow integrating RapidFire-MS with real-time informatics for high-throughput biologics screening and analysis.
PEGS Europe 2024

POSTERAdvancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Introducing the new multi-protein quantitation mass spectrometric analysis workflow tools for discovery-phase protein scientists.

POSTERMiddle-Level Approach for Blinatumomab Sample Prep and Analysis
A middle-level approach simplifies sample preparation and data analysis for the characterization of the fusion protein Blinatumomab.

POSTEROligonucleotide IP-RPLC-MS/MS for mRNA Sequence Characterization
Digested oligonucleotide IP-RPLC-MS/MS characterizes mRNA sequence, 5’ cap, and 3’ polyA tail.
CASSS 2024

POSTERAdvancements in Multi-Protein Quantitation for Host Cell Protein Discovery
Introducing the new multi-protein quantitation mass spectrometric analysis workflow tools for discovery-phase protein scientists.

POSTERCharge Variant MS for Biotherapeutic Analysis with Sampling
Charge variant mass spectrometry is used for analyzing biotherapeutics with orthogonal and fractionated sampling.

POSTERAutomated Spectrum Annotation of Released N-Linked Glycans
Released N-linked glycans are annotated and structurally disambiguated through automated spectrum analysis.

POSTEROligonucleotide IP-RPLC-MS/MS for mRNA Sequence Characterization
Digested oligonucleotide IP-RPLC-MS/MS characterizes mRNA sequence, 5’ cap, and 3’ polyA tail.

POSTERMiddle-Level Approach for Blinatumomab Sample Prep and Analysis
A middle-level approach simplifies sample preparation and data analysis for the characterization of the fusion protein Blinatumomab.
IRT 2024
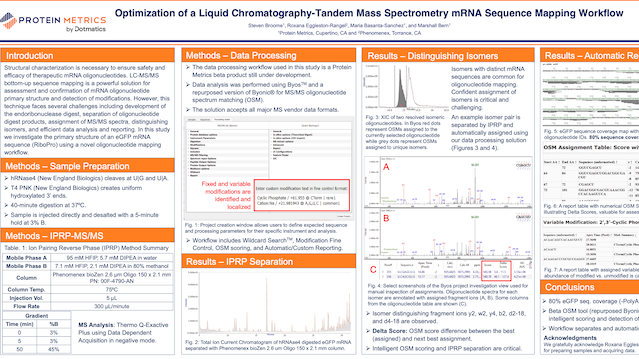
POSTEROptimized LC-MS Workflow for mRNA Sequence Mapping
A liquid chromatography-tandem mass spectrometry workflow is optimized for mRNA sequence mapping.
ASMS 2024

POSTERModeling MS1 and MS2 Effectiveness for mRNA Sequence Confirmation
Computer modeling evaluates the effectiveness of MS1 and MS2 for mRNA sequence confirmation.

POSTERDrug-to-Antibody Ratio of ADC in Biological Samples by LC-MS
The drug-to-antibody ratio of antibody-drug conjugates is determined in biological samples using microflow-liquid chromatography/HRMS.

POSTEROptimization of Disulfide Linkages in Bispecific Antibodies
Disulfide linkages in the complex cysteine-engineered stapled scFv for bispecific antibodies are optimized and characterized.

POSTERMS Label-Free Quantitation Strategies for Therapeutic Proteins
Different MS label-free quantitation strategies are assessed for therapeutic proteins.

POSTERMiddle-Level Approach for Blinatumomab Sample Prep and Analysis
A middle-level approach simplifies sample preparation and data analysis for the characterization of the fusion protein Blinatumomab.

POSTERCharge Variant MS for Biotherapeutic Analysis with Sampling
Charge variant mass spectrometry is used for analyzing biotherapeutics with orthogonal and fractionated sampling.

POSTERIntact Reconstruction for Bi-Specific Antibody Characterization
Integrating tryptic digest peptide data with intact MS data using the new Reconstruction Workflow.

POSTERNetwork Method to Build Sample-Specific Glycan Database
A network method builds a sample-specific glycan database for N-linked glycosylation from MS/MS data.
TIDES 2024

POSTERAutomated LCMS Analysis of Oligonucleotide Samples
Improve your organization’s efficiency and capability with automated LCMS analysis of synthetic oligos.
PEGS Boston 2024

POSTERAutomated Spectrum Annotation of Released N-Linked Glycans
Released N-linked glycans are annotated and structurally disambiguated through automated spectrum analysis.
Festival of Biologics 2023

POSTERAutomated Spectrum Annotation of Released N-Linked Glycans
Released N-linked glycans are annotated and structurally disambiguated through automated spectrum analysis.

POSTERReal-Time Informatics Pipeline for Biologics Screening During Expression
A real-time informatics pipeline enables the screening of biologics during high-throughput expression.
CASSS 2023

POSTEROptimized LC-MS Workflow for mRNA Sequence Mapping
A liquid chromatography-tandem mass spectrometry workflow is optimized for mRNA sequence mapping.

POSTERHis-HDX MS for Protein-Ligand Interaction Identification
Histidine hydrogen-deuterium exchange (His-HDX) mass spectrometry identifies protein-ligand interactions.

POSTERAutomated Spectrum Annotation of Released N-Linked Glycans
Released N-linked glycans are annotated and structurally disambiguated through automated spectrum analysis.
BMSS 2023

POSTERHis-HDX MS for Protein-Ligand Interaction Identification
Histidine hydrogen-deuterium exchange (His-HDX) mass spectrometry identifies protein-ligand interactions.

POSTEROrthogonal LCMS Techniques for Lysine-Conjugated ADC Characterization
Orthogonal techniques are used for LCMS characterization of a lysine-conjugated ADC.
Other Posters

POSTERNovel In Vitro Serum Stability Assay for Antibody Therapeutics
A novel in vitro serum stability assay for antibody therapeutics incorporates internal standards.

POSTERChallenges and Strategies in Glycan Analysis by MAM
Challenges and strategies are discussed in glycan analysis by MAM.

POSTERFragmentation-Free Characterization of Glycopeptides via Ion Mobility
Glycopeptides are characterized without fragmentation using ion mobility.
