SVs, considered impurities, exist at low levels and are challenging to detect and characterize. Learn about automated and flexible identification of these low abundance species

Symphogen explain how they are leveraging, through Chromeleon and Byosphere, their MS data more efficiently, and integrating data acquisition and processing tools.
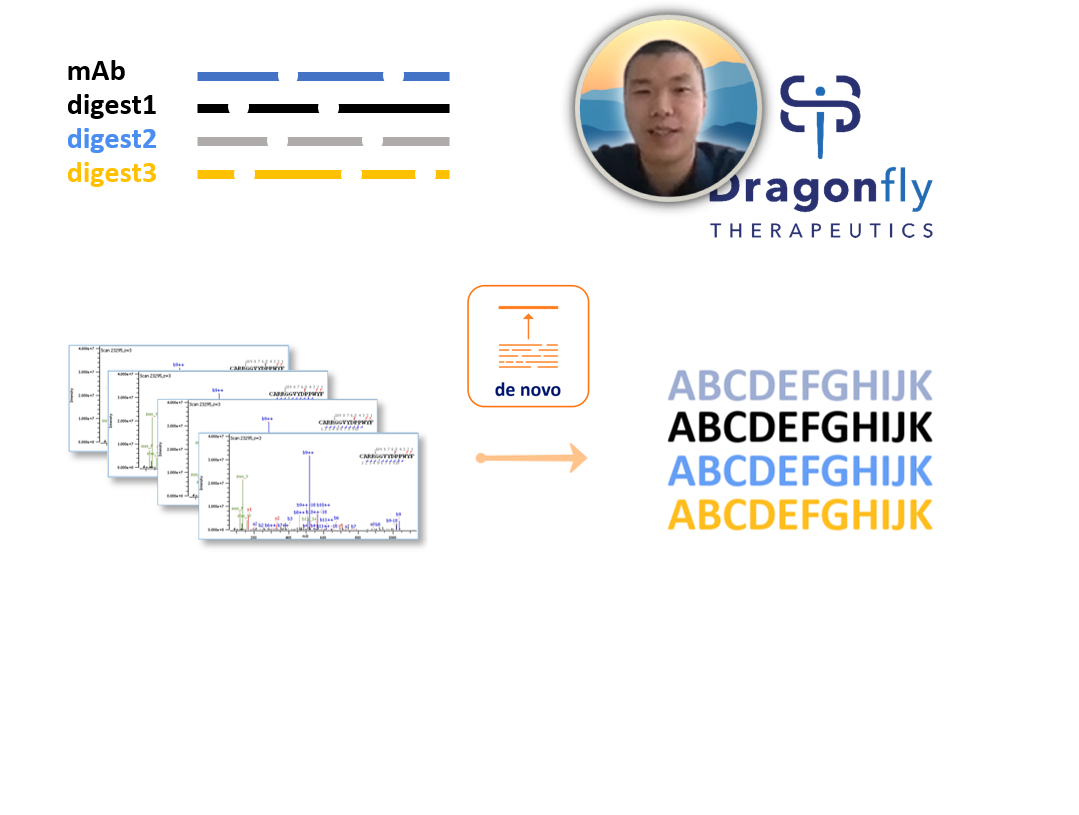
By taking de novo sequencing in house, Dragonfly have improved their turn around time, throughput, their depth of information on their mAbs as well as showing impressive cost savings
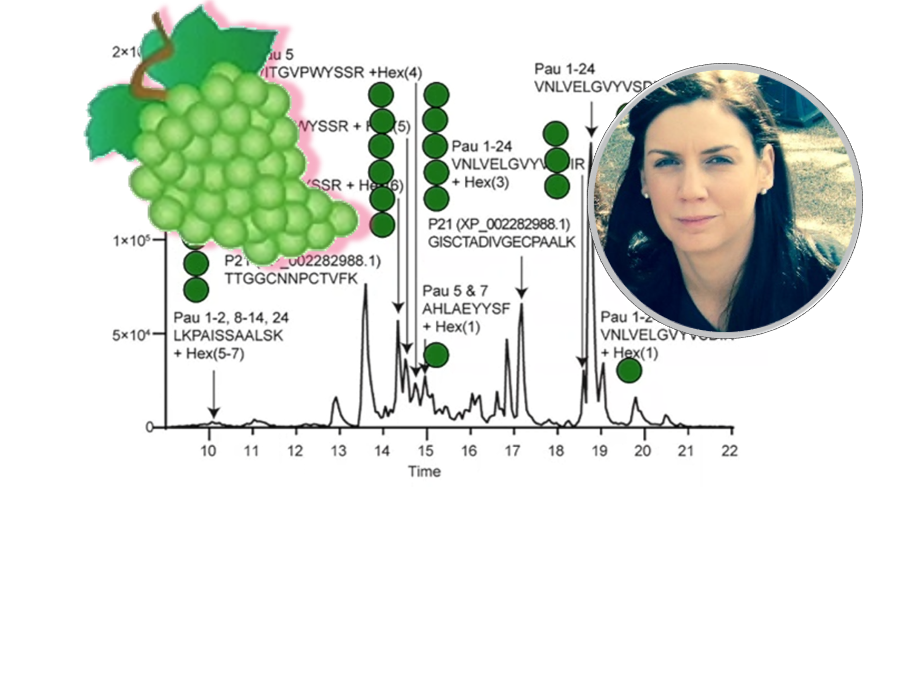
Glycoproteins have been linked to positive and negative qualities in sparkling wine, but the glycosylation profiles of sparkling wine have not been previously investigated in detail. Hear from Dr Pegg on how she has been addressing the glycoproteome analysis of sparkling wine.

For fast ranking during expression vector design and clone selection, Lonza's setup for high throughput intact MS uses Protein Metric’s parsimonious charge deconvolution algorithm.

Can sample pretreatment hide biologically important glycan species? Can Ion mobility be used to boost glycopeptide detction?

Methods for analyzing footprinting data in the context of aggregation to arrive at actionable insights
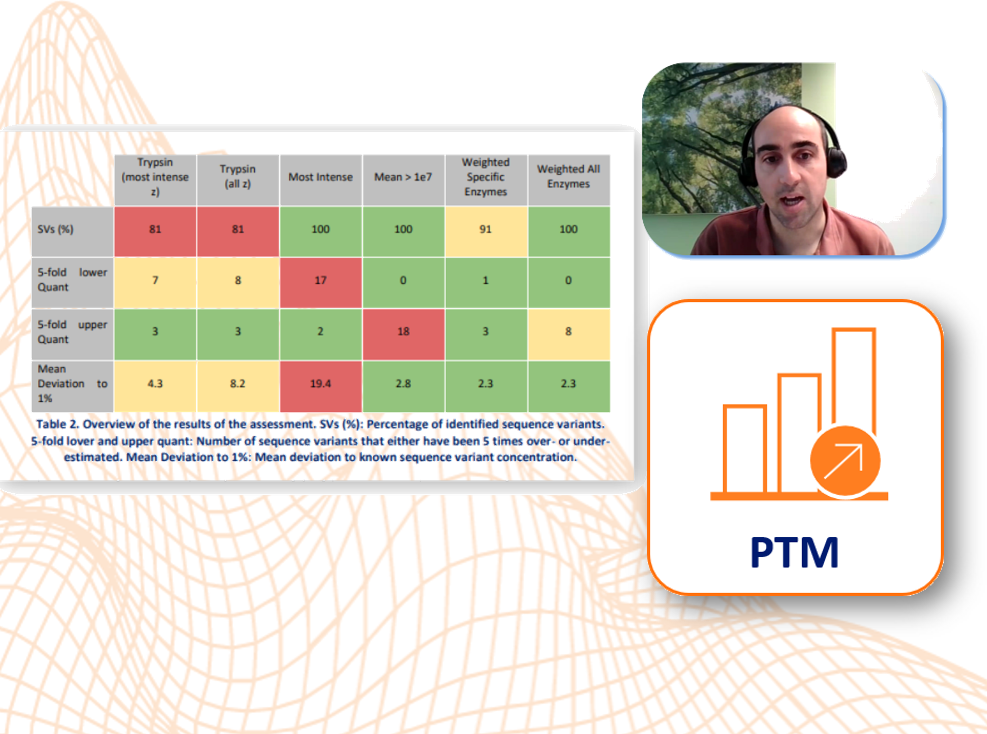
Roche’s work underscores the importance of selecting an accurate strategy for PTM analysis, and shows the application of a customized report template and dynamic columns feature to optimize label-free peptide quantitation strategies
Multi-Attribute Monitoring (MAM) in Byos® Part A – CQA Identification (和訳版)
Multi-Attribute Monitoring (MAM) in Byos® Part B – New Peak Detection & CQA Quantification (和訳版)
Rapid Identification and Quantitation of Disulfide Bonds in Infliximab – Remicade vs Remsima
Automating Sequence Variant and Post-Translational Modification Analysis (中译版)
Informative Protein Charge Variant Analysis by Native Spray Mass Spectrometry
Glyco-Analysis: Byonic™: N-Linked Glycopeptide Analysis (和訳版)
Glyco-Analysis: Byonic™: O-Linked Glycopeptide Analysis (和訳版)
Drug-to-antibody ratio quantification using native SECMS analysis of antibody–drug conjugates
Evaluation of a New Software Tool for Assisting with the siRNA Metabolite Identification by LC/MS
Automated Data Filtering for Rapid Sample Comparison of Human milk Glycoproteins
AAV Characterization: Direct Bottom-up versus Intact MS analysis Comparison (中译版)
Coagulation factor IX analysis in bioreactor cell culture supernatant
Highly Automated Analysis of Chain Shuffling in Bispecific Antibodies
How to Maintain Data Quality across Time and Mass Spectrometry Platforms
Comparability and Biosimilarity Assessment with Protein Metrics
Byosphere™ Enterprise Platform: Bringing Speed and Depth to Protein Mass Spec Analysis
Byosphere® Enterprise Platform: Manage LC-UV-MS Data Seamlessly with Byosphere®
Sciex – Characterization of a multi-specific antibody therapeutic with peptide mapping
Intact Mass: Workflow Setup for Low MW (<10 kDa) Isotopically Resolved Peptides
Intact Analysis of a Purified Therapeutic Protein Incorporating Top-Down Fragmentation
Byosphere: Custom Metadata Fields
0:50Byosphere: Intact Web Analysis
15:06Byonic: Modification Fine Control
7:45Intact Mass: Workflows Overview
3:33Intact Mass: Adding data and peak integration options
2:47Intact Mass: Deconvolution settings
3:19Intact Mass: Using .csv workflows
2:36Peptide: Creating Projects, Samples and Proteins Tabs
2:13Peptide: Processing Nodes: Byonic Intro
1:37Peptide: Processing Nodes: Byonic Details
7:48Peptide: Processing Nodes: Byologic Options
9:03Peptide: GUI, Overview, Validation, Comments, Labels
7:57Peptide: Peptide Manager Explained
10:28Chromatogram: Workflows Overview
1:57Chromatogram: Integration Set Up
6:02Chromatogram: Creating a MS2 Reference Project
3:08Chromatogram: Creating an in-silico Reference Project
5:01Chromatogram: Creating a Comparison Project
2:30Chromatogram: Baseline Anchors
2:42Chromatogram: Released Glycans Introduction
1:08Chromatogram: Released Glycans Project Creation
5:55Chromatogram: GUI & Project Interface
6:24Ligand Binding with High Res Native MS
4:24Unanticipated N- and O- Glycopeptides
3:31Byonic: Byonic Only workflow in Byos
4:40Reconstruction: Intact Reconstruction
8:02Chromatogram: Processing Biophysical (non-MS) data
5:55new Digested Oligos: Introduction
2:06new Digested Oligos: Workflow Set Up
9:46new Digested Oligos: Modifications
6:55new Digested Oligos: Investigation View
6:21new Digested Oligos: Customizing Layout
4:55new Digested Oligos: Reporting
2:54new Digested Oligos: Table View
14:12Reconstruction: Charge Variant Reconstruction
5:01アジレント、プロテインメトリックスが提供する医薬品オリゴ核酸分析の課題と解決法
59:10Reconstructing cIEF Profiles Using Peptide Mapping Data
35:05NA_UGM24: Dashboards and Deep Query
8:01Bovine milk immunoglobulin G: NeuAc to NeuGc
39:03NA_UGM24: Navigating the Future of Laboratory Automation
29:10NA_UGM24: Intact Mass Characterization of Multispecific Therapeutic Antibodies
29:01NA_UGM24: How Protein Metrics Saved Me From Carpel Tunnel
29:54NA_UGM24: A 3 body problem: Automation, Integration & Human
19:58NA_UGM24: MS analysis of intact oligonucleotides
29:37NA_UGM24: Panel Discussion – Gearing Up For Smarter Workflows
46:27Unraveling the Twin Tales of Biosimilar and Innovator Glycans
41.26Increasing Analytical Efficiency at a CDMO
18:38HDX-MS analysis of SARS-CoV-2 spike ectodomain
32:54Spotting the Outliers and New Peaks in MAM Runs Automatically
46:12JUGM 2023: 生体試料中のADCのDAR推移の分析におけるBYOSの活用例
24:06JUGM 2023: 目からうろこByos/Byonicの成り立ちから有効活用のtipsまで
28:00JUGM 2023: ByosがMulti-Attribute Method(MAM)に貢献できること
17:19JUGM 2023: ヒスチジン水素重水素交換質量分析法による生体内タンパク質・リガンドの網羅的な結合評価への挑戦
18:18Resolving Antibody Aggregation During Discovery & Development
27:29EU UGM 2023: Impurity Characterisation of modified siRNA using Byos Oligo Workflow
27:36EU UGM 2023: Enhancing the Dimensionality of Glycoproteomics
33:27EU UGM 2023: The Growing Importance and Changing Needs for Data Analysis & Informatics – US UGM
26:24EU UGM 2023: Annotation of topdown mass spectra of proteins and oligonucleotides
37:52EU UGM 2023: Byosphere assisted biopharmaceutical development
19:15EU UGM 2023: Friends with benefits: Glycoproteomics meets Chemical Biology
20:31EU UGM 2023: Introduction & State of the Union
25:53Neutrophil azurophilic granule glycoproteins – atypical glycans
54:23Mass Spectrometry Techniques Enabling Accelerated Process and Product Development of Bispecific Antibodies (中译版)
33:28WCBP 2023: Unearth the value of your complex biopharma data
14:07Tackling the Glycan Isomer Challenge for your Biotherapeutics
38:23質量分析によるオリゴヌクレオチド分析:配列の表示および自動化はもはやシーシュポスの作業(果てしない徒労)ではない
56:21High throughput screening and data analysis using Native Mass Spectrometry and Byos
34:46Monitoring System Suitability – Byosphere Dashboards (中译版)
33:49PEGS 2022 – Mining Biological Attributes with Dashboards
30:002022 Bioprocessing Summit: Autonomous Pipeline for Characterizing Biotherapeutics
28:53EU UGM 2022 – Importance of Data Analysis & Informatics in Development – Dashboards Latest
36:55EU UGM 2022 – Introduction & State of the Union
30:46EU UGM 2022 – Development of a HILIC method for detection of low abundant N-glyco-peptides
30:46EU UGM 2022 – Rapid Analysis of Oligonucleotides by High-Resolution Accurate Mass CE-MS
31:49EU UGM 2022 – LC-MS and LC-MS/MS Analyses of Adeno-Associated Viruses Anc80
18:56EU UGM 2022 – EPO analysis using ZIP Chip CE-MS and Byos Software
34:14EU UGM 2022 – Addressing MS Data Bottlenecks and Biopharmaceutical Case Studies
43:02Stanford SUMS Seminar – Connecting LC-MS data analysis with non-MS techniques
48:47Intact Profiling and Visualization of Urinary Prostate-Specific Antigen Profiles
37:51Generating Wisdom from Raw Data – The Post Genomic World
17:26The Sweet Science of Sparkling Wine
18:23Assigning function to the structure of HIV and SARS-CoV-2 glycan shields
25:08Analytical Characterization of Oligonucleotides by LC-HRMS and BYOS
47:05Three Channel Charge Deconvolution
18:47Byosphere™ Enterprise Platform – Metadata & Dashboards
19:13Harnessing Novel MS Approaches (MAM, IEX-MS) to Accelerate Biotherapeutic Development and Analysis
43:00Bringing your analysis to the enterprise: Introducing Byosphere™
30:56Ensuring Safety and Efficacy of Biotherapeutics Through In-Depth LC-MS Sequence Variant Analysis
45:57EAD: Making characterization of complex biotherapeutics routine
38:20Oligo Analysis Simplified: Get Your Answers Faster with Microchip CE-MS
51:08Bispecific MS Analysis and Cell Line Development
57:17Recent Progress in MS-Based Workflows and Case Studies from a Biopharmaceutical Development Lab
52:09Comprehensive Adeno-Associated Virus CQA Analysis with LC/MS
59:20Characterizing Next Generation Biotherapeutics with Novel Ion Trapping and Alternative Fragmentation
36:29Implementing an Enterprise Solution for MAM with LC-UV-MS
45:51Data Visualisation for Routine Host Cell Protein Analysis
37:56Oligonucleotide Characterization Using the BYOS Intact Mass Workflow
17:42Real Time Monitoring of Bioprocess & Beyond – A Process Analytical Perspective
1:01:35Automated de novo mAb Sequencing for a Modern Biotech
42:39All Tangled Up: High Resolution Neuroproteomics of the human p-Tau Interactome
34:06Keeping it all together – Bacterial Cell Envelope Structure
30:17Automated Feedback Control of Protein Characteristics in a Perfusion Bioprocess
20:15Automating data collecting and analysis in the X-ray hydroxyl radical footprinting method
19:34Rapid Intact Charge Variant Analysis with Coupled icIEF-MS
53:39Analyzing the SARS Cov-2 Glycan Shield with Mass Spec
54:22PTM Analysis – How to Marry Speed and Confidence in R&D
1:00:51How to Advance Your Host Cell Protein Analysis with Mass Spectrometry
56:09An Implementation of Vendor-Neutral Multi-Attribute Method (MAM)
56:32Native MS and Cell Line Development
24:47Driving Efficiency in Pre-Clinical Development with Automated Mass-Spectrometry Analysis
48:58Zeta 2018: Why don’t we monitor drug product profile directly?
29:04Streamlining PTM Workflows: From Sample Prep to Data Analysis
55:34HT Analysis of Bispecific Antibody Moieties and Glycosylation
51:04Model Based Algorithms for Intact Mass Analysis of Biotherapeutics
21:29Large-scale Glycopeptide Identification by Database Search
19:59ASMS 2024: Utilization of Intact Reconstruction to Characterize Bi-specific Antibodies (Video 3:03)
ASMS 2024: Assessment of different MS Label-Free Quantitation Strategies for Therapeutic Proteins
ASMS 2024: Network method for building a sample-specific glycan database for N-linked glycosylation from MS/MS data (中译版)
ASMS 2024: Determination of drug-to-antibody ratio of antibody-drug conjugate in biological samples using microflow-liquid chromatography/HRMS (中译版)
ASMS 2024: Optimization and Characterization of Disulfide Linkages in the Complex Cysteine Engineered Stapled scFv for Bispecific Antibodies (中译版)
ASMS 2024: Computer Modeling of the Effectiveness of MS1 and MS2 for mRNA Sequence Confirmation (中译版)
ASMS 2024: A Middle-Level Approach to Simplify Sample Preparation and Data Analysis for Characterization of the Fusion Protein Blinatumomab (中译版)
CASSS 2023: Optimization of a Liquid Chromatography-Tandem Mass Spectrometry mRNA Sequence Mapping Workflow (中译版)
Fragmentation-free characterization of glycopeptides with ion mobility
ASMS 2023: Orthogonal techniques for LCMS characterization of a lysine-conjugated ADC (和訳版) (中译版)
ASMS 2023: Automated Spectrum Annotation and Structure Disambiguation of Released N-linked Glycans
ASMS 2023: A Real-time Informatics Pipeline Enables Screening of Biologics during High-Throughput Expression (中译版) (Video Poster 5:10)
ASMS 2023: Investigations of a Bispecific Antibody Dimerization via Hydroxyl Radical Footprinting
ASMS 2022 – High Throughput Automation Platform of Intact Mass Spectrometry Data to Support Cell Line Development (CLD) of Multi-specific Antibodies (和訳版)
ASMS 2022 – Comprehensive site-specific glycan profiling of a protein-based flu vaccine using electron activated dissociation (EAD) (和訳版)
ASMS 2022 – Comprehensive characterization of complex linkage structures in a bispecific monoclonal antibody (mAb) using electron activated dissociation (EAD) (和訳版)
ASMS 2022 – Chromatography ‘super-resolution’: Improved HDX mapping using long gradients and time warping (和訳版)
ASMS 2022 – High-Sensitivity Charge Deconvolution Algorithm for Low-Abundance Biological Macromolecules (和訳版) (中文视频)
ASMS 2022 – Characterization of Biotherapeutics with High-Resolution Ion Mobility-Mass Spectrometry
BMSS 2022: Oligonucleotide Analysis by Mass Spectrometry: Sequence Display and Automation is no longer a Sisyphean Task (和訳版)
CASSS 2022: Continuous Monitoring of System Suitability with Byosphere® Automation and Dashboards (Video 4:57)
FOB 2022: Enabling a Multi-Attribute Method Comparison of Biosimilars
HUPO 2022: Accelerated O-glycopeptide search with isobaric filtering (Video 1:29)
HUPO 2022: N-Glycoproteomics MS/MS Data Analysis Benefits from Glycomics-lnformed Search Strategies
ASMS 2021: Analytical Characterization of Oligonucleotides by LC HRMS
ASMS 2021: Comprehensive Adeno-Associated Virus (AAV) Critical Quality Attribute Analysis with LC-MS (和訳版)
ASMS 2021: High-Throughput Product Quality Assessment of Protein Therapeutics on ZipChip-MS platform
ASMS 2021: The Utility of Nanoparticle Protein Coronas for Studying the Plasma Glycoproteome
ASMS 2021: Real Time Antibody Digestion by Rapid Flow Injection Mass Spectrometry
ASMS 2021: Kitted universal MAM: Automatable Sample Processing for all Stages of Biological Drugs
ASMS 2021: Multi-attribute Monitoring of Monoclonal Antibodies by icIEF-MS
Oligonucleotide Analysis by Mass Spectrometry: Sequence Display and Automation is no longer a Sisyphean Task (和訳版)
A Glycan Wildcard Search for Identifying Glycoprotemic Dark Matter (Video 3:07)
CASSS2020: Assaying Protein / Ligand Binding with High-Resolution Native Mass Spectrometry
ASMS2020: Assaying Protein / Ligand Binding with High-Resolution Native Mass Spectrometry
ASMS2020: Reconstructing Protein Charge Heterogeneity from a Bottom-Up Approach
ASMS2020: Unravelling Hundreds of Proteoforms of Industrial Enzymes
ASMS2020: Making LC-MS Sensibly Useful for Host Cell Protein Applications (和訳版)
ASMS2020: Ion Mobility Mass Spectrometry of Glyco- and Phospho-Peptides
Superposition of Orthogonal Methods – Intact Reconstruction from Peptide Mapping
ASMS2019: Charge Deconvolution and Automatic Sequence Matching for Oligonucleotides
Glycobiology2018: Intact & Native Mass Analysis of Glycoproteins
Determining solvent access of HNE adducted myoglobin with hydroxyl radicals generated from PLIMB
NIIMBL2019: Rapid Assessment of CQAs Using Imaged CIEF-MS for Real-Time Bioprocess Decision Making
ASMS2019: Charge Deconvolution of Surface-Induced-Dissociation (SID) Spectra of Protein Complexes
Highly Automated Analysis of Antibody Chain Shuffling in Bispecific Antibodies (和訳版)
ASMS2018: Host Cell Proteins (HCPs) in Plasma-Derived Biotherapeutics
HUPO2018: Charge Deconvolution of Hard-to-Deconvolve Mass Spectra
WCBP2018: Host Cell Proteins (HCPs) in Plasma-Derived Biotherapeutics
WCBP2018: Multi-Attribute Comparison of Innovator and Biosimilar Filgrastim
PEGS2018: Antibody Analysis with Native Mass Spectrometry and Parsimonious Charge Deconvolution
WCBP2018: Antibody Analysis with Native Mass Spectrometry and Parsimonious Charge Deconvolution
ASMS2017: Analytical Characterization and Comparison of Filgrastim and Its Biosimilar
Toward chemical footprinting at residue resolution: FPOP data analysis of IL23 – antibody binding
Assaying Protein / Ligand Binding with High-Resolution Native Mass Spectrometry
Simplified and automated analysis of the highly glycosylated therapeutic protein Erythropoietin (Video Poster 4:55)
Complex glycoprotein characterization using CE-MS and Automated Analysis
TIDES2024: Oligonucleotide Samples are Amenable to Automated LCMS Analysis
A Novel in vitro Serum Stability Assay for Antibody Therapeutics incorporating Internal Standards (Video 6:56)
CASSS 2024: Automated Spectrum Annotation and Structure Disambiguation of Released N-linked Glycans
CASSS 2024: Advancements in Multi-Protein Quantitation for Host Cell Protein Discovery copy
Peak Filtering, Peak Annotation, and Wildcard Search for Glycoproteomics
Vulnerabilities in coronavirus glycan shields despite extensive glycosylation
Optimal Dissociation Methods Differ for N- and O-Glycopeptides
Automated Antibody De Novo Sequencing and Its Utility in Biopharmaceutical Discovery
Development of an LC-MS/MS peptide mapping protocol for the NISTmAb
Parsimonious Charge Deconvolution for Native Mass Spectrometry
Biosimilarity under stress: A forced degradation study of Remicade® and Remsima™
A Multidimensional Analytical Comparison of Remicade and the Biosimilar Remsima
Glycoproteomic analysis of the secretome of human endothelial cells
Byonic™ advanced peptide and protein identification software
Implication of perturbed axoglial apparatus in early pediatric multiple sclerosis
Preview: A Program for Surveying Shotgun Proteomics Tandem Mass Spectrometry Data
Comment on “Unbiased statistical analysis for multi-stage proteomic search strategies”
Two-dimensional target decoy strategy for shotgun proteomics
Conversion of methionine into homocysteic acid in heavily oxidized proteomics samples
Glycan family analysis for deducing N-glycan topology from single MS
Automated N-glycopeptide identification using a combination of single- and tandem-MS
An embeddable molecular code for Lewis X modification through interaction with fucosyltransferase 9
Attribute Analytics Performance Metrics from the MAM Consortium Interlaboratory Study
Structure of the hepatitis C virus E1E2 glycoprotein complex
Development of Software Workflow for the Rapid Detection of Cross-Linked Dipeptides
Using New Peak Detection to Solve Sequence Variants Analysis Challenges in Bioprocess Development
Impact of Trisulfide on the Structure and Function of Different Antibody Constructs
Agilent – Host Cell Protein Analysis Using Agilent AssayMAP Bravo and 6545XT AdvanceBio LC/Q-TOF
Differential T cell immune responses to deamidated adeno-associated virus vector
Intact quantitation of cysteine-conjugated antibody-drug conjugates using native mass spectrometry
Orthogonal Comparison of Analytical Methods by Theoretical Reconstruction from Bottom-up Assay Data
3:03Dragonfly Therapeutics – De Novo Sequencing
6:39Immuto Scientific – PLIMB – Plasma Induced Modification of Biomolecules
4:45Unravelling the glycosylation of the SARS-CoV-2 spike protein
7:02Unexpected Modifications in Cell-Line Characterization
7:23Automated Data Filtering for Rapid Sample Comparison of Human Milk Glycoproteins
7:01Rapid Detection of Cross-Linked Dipeptides
2:38Chemical probing provides insight into the native assembly state of a bacterial microcompartment
4:20Sample Preparation Matters for Peptide Mapping to Evaluate Deamidation of Adeno-Associated Virus Capsid Proteins Using Liquid Chromatography-Tandem Mass Spectrometry (中译版)
3:31Rapid Detection of Cross-Linked Dipeptides
2:38BEBPA 2023 Host Cell Protein Conference – In Review
18:17Automated Characterization of Biotherapeutics via Hardware, Software and Chemistry
10:42Unanticipated N- and O- Glycopeptides
3:31Optimization of a Label-Free Peptide Quantitation Strategy for Analysis of Therapeutic Proteins
6:25Comparative Analysis of Herceptin N-Linked Glycosylation by HILIC-FLD and LC- MS/MS Methods
4:16Advanced Techniques in Protein Glycosylation Analysis Using Mass Spectrometry
6:26A Novel in vitro Serum Stability Assay for Antibody Therapeutics incorporating Internal Standards
6:56Utilization of Intact Reconstruction to Characterize Bi-specific Antibodies
3:03Insights 2: Quantifying Aspartic Acid Isomerization in Byos® (和訳版)
Insights 3: Intact Deconvolution with Isotopic Resolution, Intact Reconstruction in Byos® (和訳版)
Insights 4: Dynamic Columns, XIC Area Summed isoX Normalized in Byos® (和訳版)
Insights 8: Mapping In Silico Peptides, Intact Monoisotopic Mass Visualization in Byos®
Insights 9: Delta Mod Score for Variant Analyses in Byos® and Byonic™
Insights 10: Intact Mass Workflows, Automated Annotations in Deconvoluted Spectra in Byos®
Insights 11: Validating Glycopeptide Results, MSMS Filtering in Byonic™
Insights 12: Accelerating Sequence Variant Analysis with Byos® Validators
Insights 17: Controlling how protein homology is represented in Byos
Insights 24: Quantification of Modifications in the Peptide Workflows
Insights 25: Oligonucleotide and Impurity UV-MS Quantitation

